About Us
LQMS is a leading provider of ERP solutions in the region, offering innovative solutions that drive production efficiency, cost-effectiveness, and timely project completion. The company's team of qualified professionals possesses the knowledge and expertise to bridge the gap of conformity assessment bodies by demonstrating competence and capability. LQMS's ERP solutions are highly customizable, making them an ideal choice for businesses looking to streamline their operations and enhance efficiency.
LQMS Software Solutions LLC is a software development company headquartered in Dubai, UAE. LQMS has branch offices in India, and Philadelphia, Pennsylvania, USA. Our company is a comprehensive provider of Enterprise Resource Planning (ERP) solutions in the Gulf Cooperative Council (GCC) region exclusively for conformity assessment bodies in the field of Testing, Inspection, and Certification. We maintain a team of professionals with a core competency of expertise in quality management, compliance statutes, and regulatory organizations.
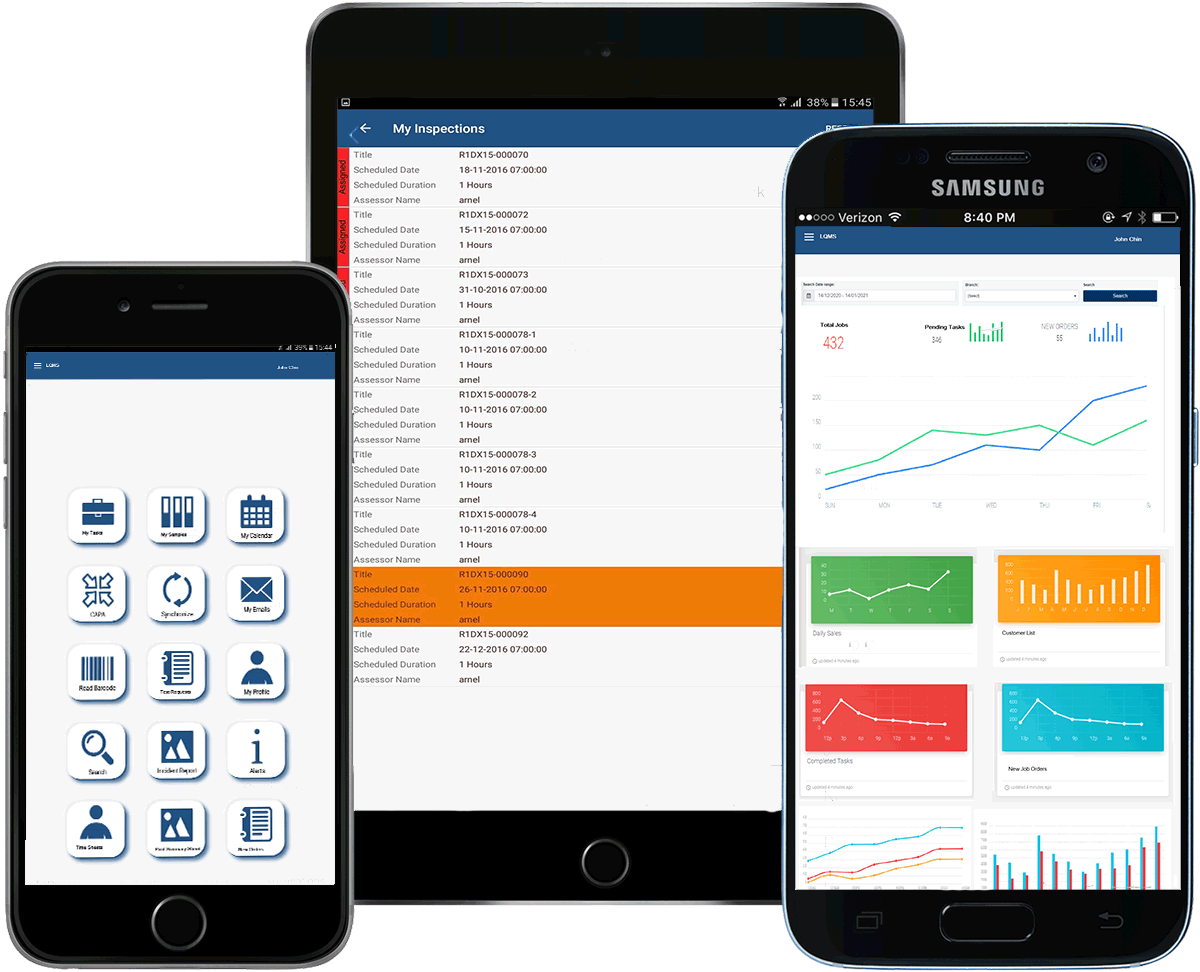
The company specializes in Laboratory Information Management Systems, Integrated Electronic Quality Management Solutions, and Accreditation and Certification Management Systems. We also provide turnkey products for Human Resources, Accounting, and Customer Resource Management (CRM) systems. Our original approach to developing innovative answers for every project ensures production efficiency, cost-effectiveness, and on-time completion for clients.
We have an extensive referenceable client base in the GCC and international geographies. Our clients include material testing labs, inspection companies, certification bodies, corporate organizations, government entities, and private hospitals.
LQMS's team of qualified professionals possesses a deep understanding of the challenges businesses face in regulated environments. The team leverages this knowledge and expertise to bridge the gap of conformity assessment bodies by demonstrating competence and capability. This approach has helped LQMS to provide innovative solutions that drive excellence in challenging, regulated environments.
Read More